Test for Nitrate Ions
The halogens are the. This has a characteristic choking smell.
Sulfuric acid cannot be used because it contains sulfate ions - these would interfere with the second part of the test.
. Lead II ion Pb2. Dissolve a small sample of the solid salt you are. The halogens are the.
Observe and record the colour of. This is done by. Firstly add aqueous NaOH to your unknown substance.
Add a few drops of dilute nitric acid to the sample. Testing for Metal ions by burning. A test using silver nitrate.
For example when silver nitrate is added to a solution containing chloride ions. This test has to be done in solution. If the litmus paper turns blue the gas is ammonia and the test for nitrate ions is positive.
This can be identified by testing it with damp red litmus paper which will turn blue in its presence. Silver ions react with the halide ions to form precipitates of silver chloride silver bromide or silver iodide which deepen in colour from white cream then. Add a few drops of dilute silver nitrate solution.
Iii About 3 cm 3 of distilled water is added and the mixture is boiled. To test for halide ions. To test for halide ions.
Add a few drops of dilute nitric acid to the sample. Add the nitrate to sodium hydroxide solution then add powdered aluminium. The aluminium reduces the nitrate ion NO 3- to an ammonium.
Testing for halide ions. Silver nitrate dilute nitric acid The nitric acid reacts with and removes other ions that might also give a confusing precipitate with silver nitrate. Nitrate ions NO3- can be detected by reducingthem to ammonia.
Then add aluminium powder or foil and proceed to heat the mixture strongly. The aluminium powder is a powerful reducing agent and converts the nitrate ion NO3- into ammonia gas NH3. Adding sodium hydroxide solution then aluminium powder or foil heating strongly If nitrate ions are present ammonia gas is given off.
Reacting with sodium chloride. Their ions are called halide ions eg. Nitrate in solution NO3 make alkaline with sodium hydroxide solution then add aluminium foil or Devardas alloy and warm carefully result is Ammonia gas is given off test with moist red litmus.
I found two versions of test for nitrate ions. Ii A dropper is used to add about 1 cm 3 of sodium chloride solution into the test tube. A white precipitate forms if sulfate ions are present.
1 boil the suspected nitrate with sodium hydroxide solution and fine aluminium powder Devardas Alloy the fumes contain ammonia which turns red litmus blue. A test using silver nitrate. There is another test for nitrates that I forgot to tell in this video - take salt NaOH aluminium foil and heat it if ammonia is evolved salt could be ni.
The nitrate ion is reduced by the aluminium and ammonia gas is given off. I About 2 cm 3 of lead II nitrate solution is poured into a test tube. Common anion tests - testing for nitrate ions in solution.
Test for halide ions. Sulfuric acid cannot be used because it contains sulfate ions - these would interfere with the second part of the test. If nitrate ions are present they will be reduced to ammonia giving off ammonia gas.
Ag aq Cl-aq AgCls. Add a few drops of dilute nitric acid then a few drops of silver nitrate solution. If you start from a solid it must first be dissolved in pure water.
A test using silver nitrate. It also turns damp red litmus paper or damp universal indicator paper blue. Testing for halide ions.
The solution is acidified by adding dilute nitric acid. Sodium Na burns giving yellow flame. Use damp red litmus paper to test the gas.
Observe and record the colour of any precipitate that forms. We can test for the halide ions the ions formed from the group 7 elements using dilute nitric acid followed by silver nitrateWe add dilute nitric acid to remove any carbonate or sulfate ions which would give a false positive result. You can test for them using silver nitrate solution.

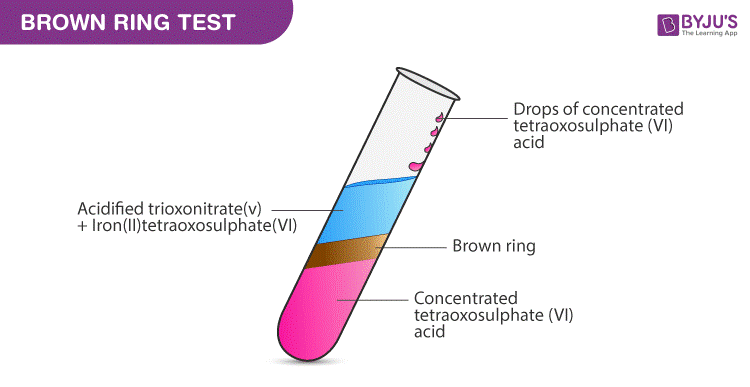
Brown Ring Test Nitrate Test Procedure Reactions On Byju S

Question Video Testing For The Presence Of Nitrate Ions Using The Brown Ring Test Nagwa

Iit Jee Individual Test Nitrate Ion No3 Offered By Unacademy

Lesson Explainer Tests For Anions Nagwa

0 Response to "Test for Nitrate Ions"
Post a Comment